Health News: US FDA Approved the 1st Home HIV Test
The US Food and Drug Administration (FDA) announced on Tuesday that is has approved the first over the counter HIV test kit that allows Americans to test, in the privacy of their own homes, whether they are infected with the virus that causes AIDS. About one in five HIV-positive people do not know they are infected with it, and may pass on the virus unknowingly.
In the Philippines historically has had a relatively low HIV rate, which experts say is particularly surprising given that condom use is discouraged by the powerful Philippines Catholic church. Experts have argued for years over why the Philippines rate is so low, with far less than 1% of the country’s 95 million people believed to have HIV or AIDs, and less than 350 new cases as recently as 2007.
But officials have seen an unexpected surge in cases in the past several years, with a more than five-fold increase between 2007 and 2011, with 2,349 new cases last year. The total number of cases as of April 2012 is 9,396, compared to just 3,061 in 2007. The Philippines is now recording seven new HIV infections a day, versus one a day before 2007, making it one of only seven countries worldwide where HIV infection rates continue to rise rapidly
Government epidemiologists have warned that by 2015, the total number of HIV cases in the Philippines could reach 45,000 from the reported 7,000 cases in 2011.
The test is a potentially exciting development as it provides an opportunity to increase the number of people who report for HIV testing (existing testing involve going to a clinic and having blood tests, which can put many people off).
The availability of convenient home-testing may encourage more people who think they may be infected to take a test. It is important to stress that, although it performs well, the test is not 100% accurate. A positive test result does not mean that the person definitely has HIV and the results need to be confirmed through further laboratory testing by healthcare professionals. A negative result does not mean that the person is definitely clear, and they may need to be re-tested at a later stage.
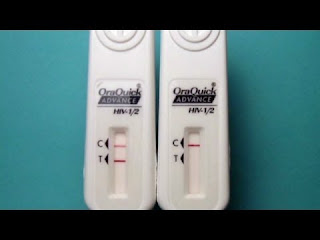
Home HIV Test
The test is called the OraQuick In-Home HIV test, and is made by the US company OraSure. It is the first over-the-counter, self-administered HIV test to be approved by the US regulator, the Food and Drug Administration (FDA). The test is now licensed to be sold to anyone aged 17 and older in shops or online in the US, although the manufacturer has yet to make it available or set a price.
The test works by detecting antibodies to the HIV virus. To do the test, a person takes a swab of their upper and lower gums and places this in a tube with a developer liquid for 20 to 40 minutes. If two lines then appear on the test stick, this indicates a positive test result. It means that antibodies against HIV were detected and the virus may be present. If only one line appears, the test result is negative.
The test is a preliminary (screening) test and results must be confirmed through follow-up testing by healthcare professionals. This is because no test is perfect, and the test may have both false-positive and false-negative results. In the US, OraQuick Consumer Support Centre will be available by telephone, with counsellors available 24 hours a day to answer questions and provide local referrals for follow-up testing and care.
Home HIV Test Aimed
The test is aimed at people who would not otherwise have an HIV test, such as those who are reluctant to go to their doctor to be tested. Some of these people may test positive for the disease using the home test, and it is hoped this result will encourage them to seek appropriate medical care and therefore reduce transmission of the disease.
Research
Studies have shown that the test correctly identified 92% of people who were carrying the HIV virus. This means that 8% (about 1 in 12 people) with the virus were missed by the test. The FDA stresses that testing negative does not mean that a person definitely does not have HIV. It also says that the test is not reliable for detecting HIV infection in the first three months after acquiring it, and that even after this it does not pick up 100% of infections.
The FDA says that people who engage in behaviours that put them at increased risk of getting HIV, such as having unprotected sex with new partners or injecting illegal drugs, should be re-tested for HIV on a regular basis.
Studies showed that 99.98% of people who did not have the virus had the correct negative test result. This means that 0.02% (or about one in 5,000 people) without HIV falsely tested positive. This illustrates that people who have a positive test still need to go to their healthcare provider for the diagnosis to be confirmed with further tests.